Current Projects
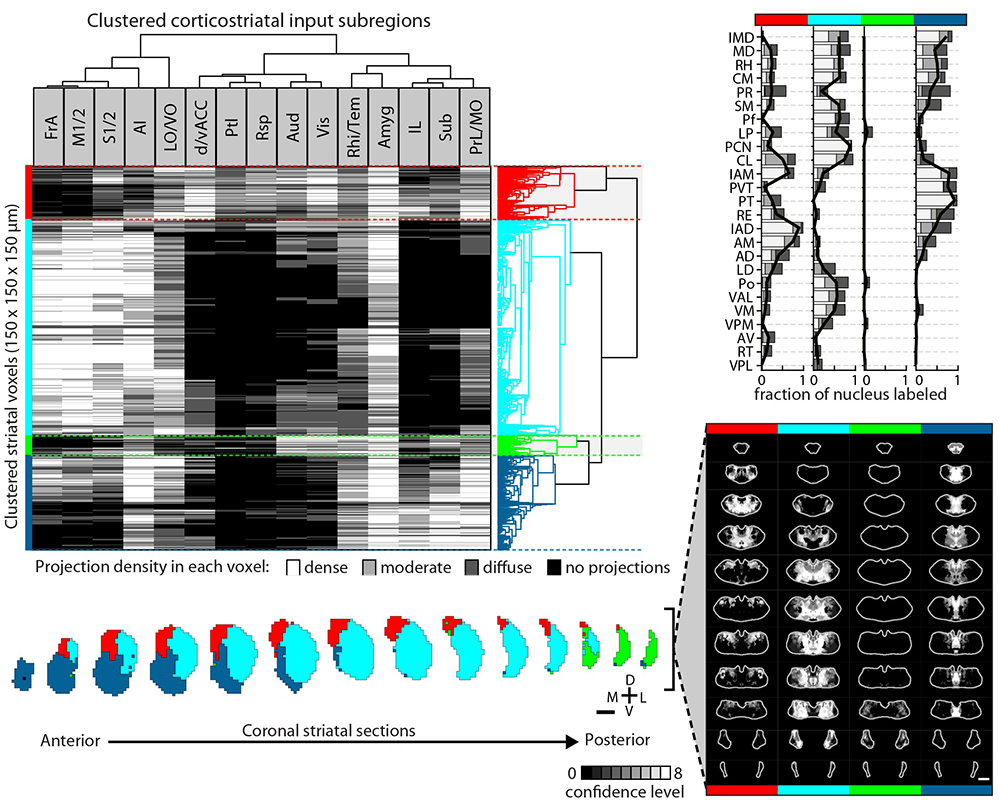
Striatal subdivisions based on cortical input convergence.
↗ Hunnicutt et al. (2016)
Electrophysiological readout from optogenetic stimulation of a thalamocortical circuit.
↗ Birdsong, Jongbloets et al. (2019)
Thalamo-Cortico-Striatal Circuitry
Thalamo-cortico-striatal circuits provide an important neural substrate for the regulation of decision-making, action selection and initiation, as well as motor learning. In order to study these circuits in the context of behavior one must understand how they are built up. In our recent work, we comprehensively mapped thalamo-cortical axonal projections and delineated potential functional thalamic subregions based on the cortical projection profile. By integrating multiple connectomic datasets, we further obtained comprehensive maps of the entire thalamo-cortico-striatal loop and illustrated the functional subregions of the mouse striatum based on the excitatory inputs. This work also demonstrated the power of biology-driven, novel computational algorithms used to re-analyze existing large datasets and extract novel biology.
Analogous to genetic screening, these comprehensive connectivity maps provide unique opportunities to investigate potentially novel and/or underappreciated specific thalamo-cortico-striatal circuits that are involved in particular behavioral processes. For example, in our 2019 eLife paper, we identified key components of the thalamo-cortico-striatal loop involved in affective pain-processing that are directly modulated by opioids. Currently, we are undertaking investigations of micro-circuits and cell types in key cortical regions based on the thalamo-cortico-striatal maps. Bart, Yang, and Ian are currently spearheading this project.
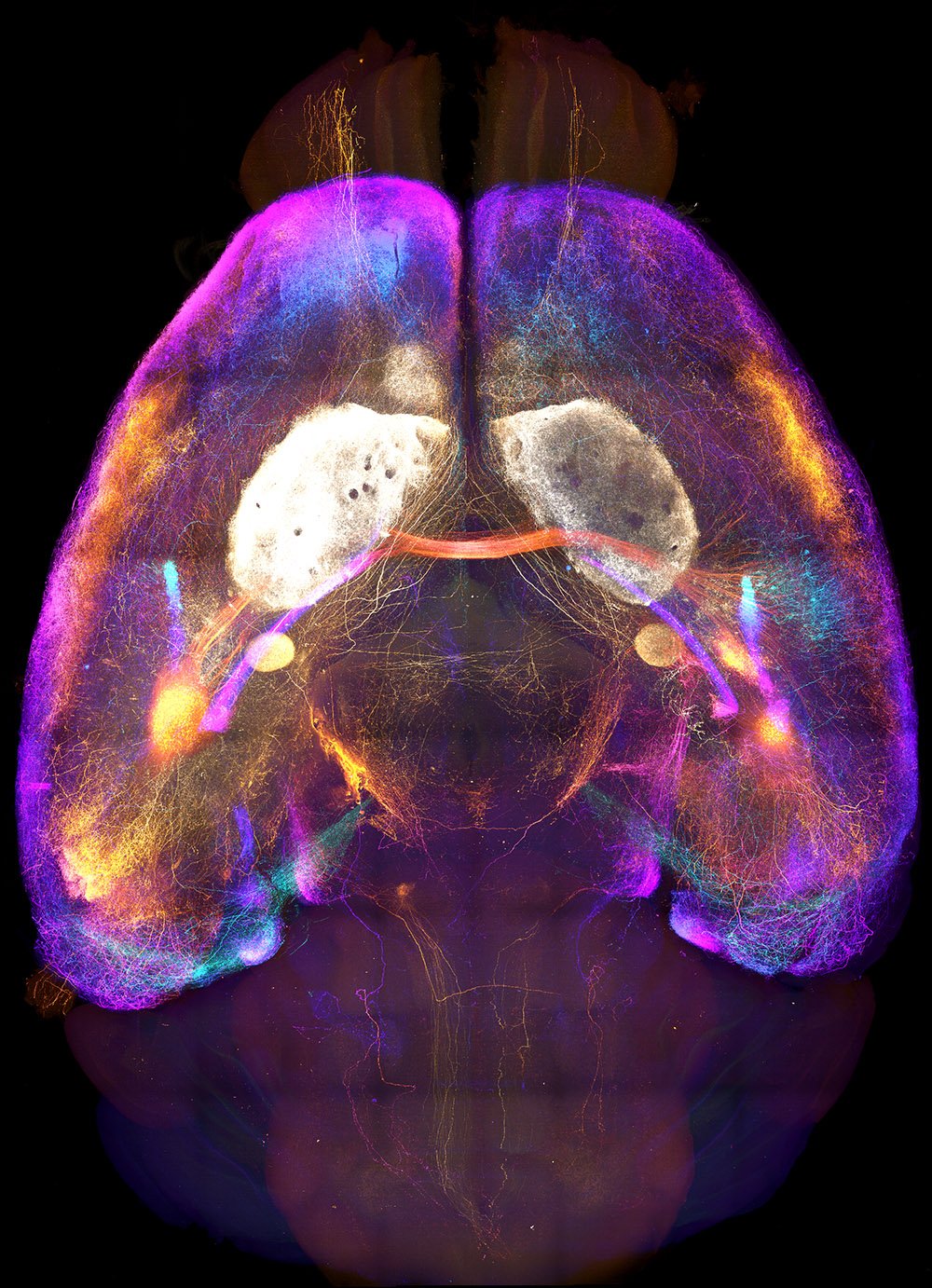
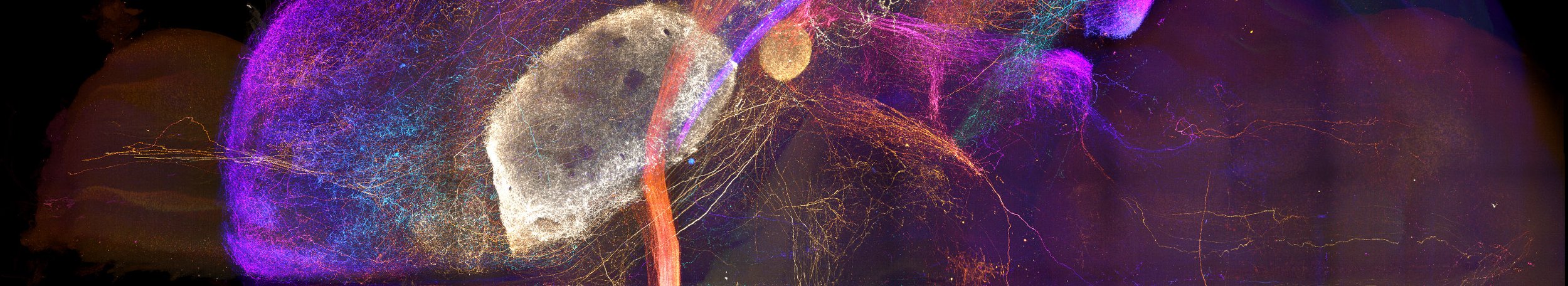
Axonal projections from the amygdala imaged in a cleared brain sample.
Whole Brain Connectomes
The brain is greater than the sum of its parts, yet it is often studied one region, circuit, or cell type at a time. Mapping axonal connections at the scale of the entire brain is an essential step toward integrating knowledge gained through more tightly focused studies. In particular, many brain regions that exert a pivotal impact on behavior exhibit broad connectivity with the rest of the brain. Our comprehensive anatomical mapping of thalamo-cortico-striatal projections laid a core foundation for us to expand our efforts to construct projectomes for other key brain regions. Now, Mike is leveraging recent advances in whole-brain optical clearing, light-sheet microscopy, and computational power to bring mesoscopic connectome work to the next level.
High resolution image of transgenically and immunohistochemically labeled cell bodies in the locus coeruleus.
Neuromodulation
Neuromodulators like norepinephrine and dopamine alter activity throughout the brain leading to dramatic changes in behavior. Not surprisingly, their signaling capabilities are as varied as the environmental changes they allow animals to respond to. However, under many conditions, the mechanistic details of neuromodulation remain a mystery. Several of the current Mao Lab projects aim to address different aspects of this biological unknown using anatomical and in vivo imaging approaches. Landon is leading these projects.
In vivo fluorescence lifetime images of barrel cortex neurons expressing a cAMP sensor (left) and a PKA sensor (right).
Norepinephrine-containing fibers from the locus coeruleus in the frontal cortex.
In vivo fluoresence lifetime images of barrel cortex neurons expressing a cAMP sensor (left) and a PKA sensor (right).
Technology Development
This part work is conducted in close collaboration with Dr. Haining Zhong’s lab.
Fluorescent sensors
Harnessing our previous experience making genetically-encoded calcium indicators (Mao et al., 2008, Tian et al., 2009 ), we have recently been focused on developing improved cAMP and PKA sensors that allow direct monitoring of cellular responses in neuromodulation and are compatible with longitudinal, single cell resolution in vivo imaging. We have recently developed tAKAR and cAMPFIRE for imaging PKA and cAMP in vivo, respectively. We are making these tools and reagents widely available.
Protein labeling techniques that enable imaging endogenous synaptic proteins in vivo
To label and image synaptic proteins in their endogenous level, we have developed two complementary approaches: a mouse genetics approach and a CRISPR/Cas9-based method.
Using endogenous labeling via exon duplication (ENABLED) for labeling synaptic protein without over-expression, we generated mouse lines labeling the excitatory postsynaptic marker PSD-95 with flexibility and high imaging contrast (Fortin, 2014). Using these lines, we imaged, for the first time, molecularly-defined excitatory shaft synapses, and determined the organization and dynamics of excitatory shaft synapses in cortical interneurons in vivo, thus, providing insights regarding cortical excitation-inhibition balance (Melander et al., 2021). We are working to further improve these lines, and we are generating more lines of the synaptic proteins.
While genetically modified mouse lines are powerful regents, they are costly and time consuming to make. We also developed CRISPIE (CRISPR-mediated insertion of exon) for protein labeling. While other methods target exons as the integration sites for fluorescent protein labels, the key advantage of CRISPIE is that it targets introns. CRISPIE results in error-free mRNA and can be used for N- and C-terminal tagging, multiple fluorescent proteins, multiple animal species, various transfection methods, diverse cellular proteins, and can be made reversible. We hope this technology will enable researchers to introduce somatic knock-in mutations to organisms in an efficient and cost-effective way. We are optimizing CRISPIE to improve the editing efficiency of the method.